Summary
Estimated dietary acid load (EDAL)
A food’s acid load represent its content in acidifying and alkalizing nutrients, which some researchers think can affect the pH of the blood.
The three most common methods to determine a food’s estimated dietary acid load (EDAL) are the potential renal acid load method (PRAL) and two net endogenous acid production methods: the Frassetto method (NEAP-F) and the Remer method (NEAP-R).
In PRAL, EDAL is increased by protein and phosphorus and decreased by calcium, magnesium, and potassium. NEAP-R multiplies PRAL by the estimated organic acid excretion, which is based on the estimated body surface area. Finally, in NEAP-F, EDAL is simply increased by protein and decreased by potassium.
Those three methods share two characteristics: they consider that protein is acidifying and potassium alkalizing. But why?
Acidity and alkalinity
A proton and an electron hold equal electric charges: positive for the proton, negative for the electron. If an atom has an uneven number of protons and electrons, it is electrically charged and called an ion. A positively charged ion is a cation. A negatively charged ion is an anion.
A hydrogen atom (H) is composed of a proton and an electron. In other words, a free proton is a hydrogen atom without its electron: it’s a hydrogen cation (H+).
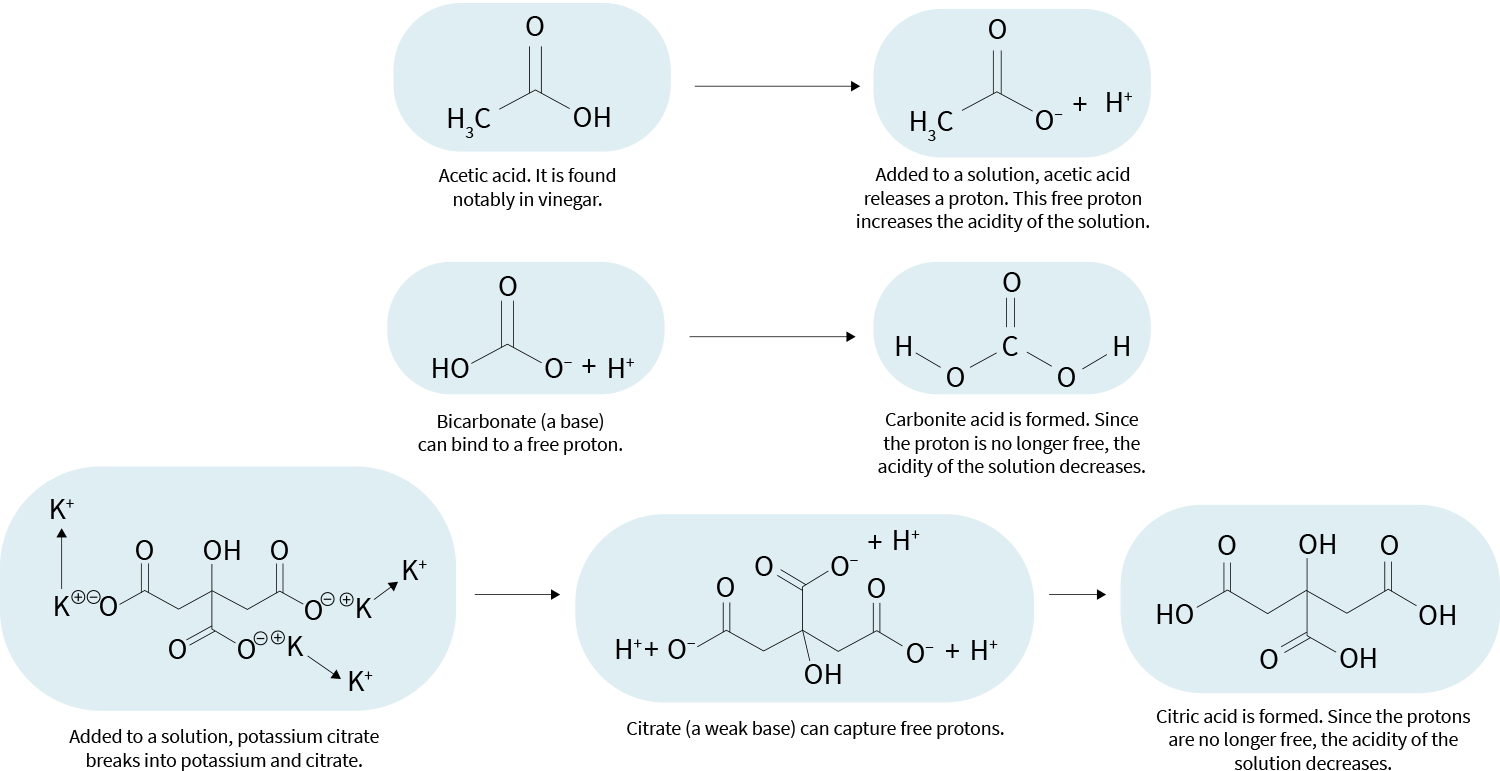
“Acid” can mean different things in different contexts. Here, it means a molecule that, when added to a solution, can release a proton. Molecules that readily do so are called strong acids.
“Base” is a negatively charged molecule that can bind to free protons, thereby reducing the acidity of a solution. An acid that releases its proton becomes a base (a “conjugate base”, to be precise), and so becomes able to capture free protons.
An acidic solution is therefore composed of molecules that keep capturing and releasing protons. A state of balance is reached, in which the number of free protons stays about the same. This number determines the acidity of the solution.
Conversely, the alkalinity of a solution is determined by its number of bases (the molecules that can capture free protons). In other words, a solution’s alkalinity is this solution’s ability to resist acidification.
Protein and potassium
In food, potassium usually exists as several cations attached to an anion — primarily citrate or bicarbonate. In the body, the citrate and bicarbonate separate from the potassium; they can then act as bases and capture free protons, making their environment less acidic. Dietary potassium is therefore considered alkalizing, despite not being itself a base.
Acids and bases — simplified examples
Dietary protein, on the other hand, is considered acidifying. The main reason is believed to be that the metabolism of methionine and cysteine (two sulfur-containing amino acids) can produce sulfuric acid, which can release protons. These released protons (free protons) make their environment more acidic.
For the sake of simplicity, the calculations used to estimate dietary acid loads treat all proteins as equal. Yet if proteins are acidifying mostly because of their methionine and cysteine content, some proteins will be more acidifying than others. For instance, there’s a lot more methionine and cysteine in milk protein and rice protein than in pea protein and soy protein, as this table shows.
Health claims
There are claims that acidifying diets erode bones (this is called the acid-ash hypothesis[1]) and promote cancer, diabetes, and kidney stones. Let’s have a look at the evidence.
Bone health
Dietary protein is considered acidifying, yet bone health doesn’t appear to suffer from higher protein intakes. In RCTs, higher protein intakes had a positive effect on lumbar-spine BMD and a neutral effect on hip BMD, femoral-neck BMD, calcium balance (i.e., calcium absorbed vs. calcium excreted), and markers of bone breakdown (e.g., urinary N-telopeptide [NTX]). Similarly, in prospective cohort studies, higher protein intakes were associated with either a neutral effect or a positive effect on BMD and the risk of bone fracture.[2][3]
If dietary protein is considered acidifying, citrate and bicarbonate are considered alkalizing. So what effect do they have on bone health? In a 2015 meta-analysis of 14 RCTs that tested potassium citrate or potassium bicarbonate, NTX (assessed in 7 RCTs) was lowered, indicating a possible bone benefit, yet BMD (assessed in 2 RCTs, both of which used potassium citrate) was unaffected.[4] Moreover, one of the two RCTs that assessed BMD also tested an increased intake of fruits and vegetables (about +300 g/day), and this theoretically alkalizing intervention also left BMD unaffected.[5]
Despite protein being acidifying, a considerable body of evidence suggests that higher protein intakes do not harm bone health (and may even improve it). In clinical trials, supplementation with potassium bicarbonate and potassium citrate (two alkalizing compounds) improved one marker of bone breakdown — but not bone mineral density.
Cancer
Some observational studies have associated EDAL and cancer risk, but there exist only one or two studies by type of cancer (breast cancer,[6][7] lung cancer,[8], pancreatic cancer,[9] and glioma,[10][11]), which doesn’t make for solid evidence. Additionally, because diets with a higher EDAL tend to be higher in animal-based foods and lower in plant-based foods, there are many confounders at play. In other words, a diet rich in fruits and vegetables may lower cancer risk for reasons unrelated to its lower EDAL. For instance, a meta-analysis of 7 observational studies (which is summarized here) found that anthocyanins might decrease the risk of colon cancer.[12]
Observational studies have linked a higher dietary acid load to a higher risk of a few types of cancer, but this evidence is preliminary (the studies are few and the potential confounders numerous).
Type 2 diabetes
Some observational studies have also associated EDAL and the risk of type 2 diabetes,[13][14] but as we noted above, there are many potential confounders, and so the evidence is weak. Furthermore, relevant clinical trials are far more equivocal. For instance, increased protein intakes haven’t led to increased insulin resistance in RCTs.[15][16] Whether protein increases or decreases insulin resistance is still a subject of debate,[17] but this debate is mostly about protein potentially increasing insulin resistance because of its BCAAs,[18] not because of is acidifying properties. As for compounds with alkalizing properties, the few trials that tested sodium bicarbonate or potassium bicarbonate found no effect on markers of glycemic or insulin control.[19][20]
Observational studies have linked a higher dietary acid load to a higher risk of type 2 diabetes, but this evidence is preliminary (the studies are few and the potential confounders numerous). In clinical trials, eating more protein (which is acidifying) didn’t worsen insulin resistance, whereas supplementation with sodium bicarbonate or potassium bicarbonate (two alkalizing compounds) didn’t improve insulin resistance.
Kidney stones
Finally, EDAL has been studied in the context of kidney stones. What we eat does seem to affect urine pH,[21] and a lower urine pH is generally thought to increase the propensity of certain solutes (particularly uric acid) to crystallize, thereby increasing the risk of kidney-stone formation in susceptible individuals. Supplementation with alkaline compounds (e.g., potassium citrate) decreased this risk in several trials, but these trials had issues, including incomplete outcome data and a frequent lack of randomization.[22] Moreover, even if citrate does decrease the risk of kidney-stone formation, this effect may be unrelated to citrate’s effect on urine pH.[23]
A higher dietary acid load can reduce the pH of the urine, potentially increasing the risk of kidney stones. In clinical trials, potassium citrate was shown to prevent kidney stones, but these trials had many flaws. Moreover, even if citrate does prevent kidney stones, it may do so for reasons unrelated to its being alkalizing.
References
- ^Rachel Nicoll, John McLaren HowardThe acid-ash hypothesis revisited: a reassessment of the impact of dietary acidity on boneJ Bone Miner Metab.(2014 Sep)
- ^Taylor C Wallace, Cara L FrankenfeldDietary Protein Intake above the Current RDA and Bone Health: A Systematic Review and Meta-AnalysisJ Am Coll Nutr.(2017 Aug)
- ^Shams-White MM, Chung M, Du M, Fu Z, Insogna KL, Karlsen MC, LeBoff MS, Shapses SA, Sackey J, Wallace TC, Weaver CMDietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis FoundationAm J Clin Nutr.(2017 Jun)
- ^H Lambert, L Frassetto, J B Moore, D Torgerson, R Gannon, P Burckhardt, S Lanham-NewThe effect of supplementation with alkaline potassium salts on bone metabolism: a meta-analysisOsteoporos Int.(2015 Apr)
- ^Helen M Macdonald, Alison J Black, Lorna Aucott, Garry Duthie, Susan Duthie, Rena Sandison, Antonia C Hardcastle, Susan A Lanham New, William D Fraser, David M ReidEffect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trialAm J Clin Nutr.(2008 Aug)
- ^Ronco et alDietary acid load and breast cancer risk: A case-control study in UruguayEuropean Journal of Cancer.(2020-10)
- ^Yong-Moon Mark Park, Susan E Steck, Teresa T Fung, Anwar T Merchant, M Elizabeth Hodgson, Jean A Keller, Dale P SandlerHigher diet-dependent acid load is associated with risk of breast cancer: Findings from the sister studyInt J Cancer.(2019 Apr 15)
- ^Alvaro L Ronco, Wilner Martínez-López, Juan M Calderón, Wilson GolomarDietary acid load and lung cancer risk: A case-control study in menCancer Treat Res Commun.(2021)
- ^Li-Wei Shi, Yi-Lin Wu, Jie-Jun Hu, Peng-Fei Yang, Wei-Ping Sun, Jian Gao, Kang Wang, Yang Peng, Jing-Jing Wu, Guo-Chao ZhongDietary Acid Load and the Risk of Pancreatic Cancer: A Prospective Cohort StudyCancer Epidemiol Biomarkers Prev.(2021 May)
- ^Alireza Milajerdi, Mehdi Shayanfar, Sanaz Benisi-Kohansal, Minoo Mohammad-Shirazi, Giuve Sharifi, Hadi Tabibi, Ahmad EsmaillzadehA Case-Control Study on Dietary Acid Load in Relation to GliomaNutr Cancer.(2021 Jul 29)
- ^Mousavi et alRelationship between dietary acid load and glioma: A case-control studyQom Univ Med Sci J.(2009-03)
- ^Xin Wang, De-Yi Yang, Liu-Qing Yang, Wen-Zhi Zhao, Li-Ya Cai, Han-Ping ShiAnthocyanin Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Observational StudiesJ Am Coll Nutr.(2019 Jul)
- ^Parvin Dehghan, Mahdieh Abbasalizad FarhangiDietary acid load, blood pressure, fasting blood sugar and biomarkers of insulin resistance among adults: Findings from an updated systematic review and meta-analysisInt J Clin Pract.(2020 Apr)
- ^Jessica C Kiefte-de Jong, Yanping Li, Mu Chen, Gary C Curhan, Josiemer Mattei, Vasanti S Malik, John P Forman, Oscar H Franco, Frank B HuDiet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studiesDiabetologia.(2017 Feb)
- ^Zhangping Yu, Fengwei Nan, Leslie Yingzhijie Wang, Hua Jiang, Wei Chen, Yu JiangEffects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trialsClin Nutr.(2020 Jun)
- ^Lukas Schwingshackl, Georg HoffmannLong-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysisNutr J.(2013 Apr 15)
- ^A Rietman, J Schwarz, D Tomé, F J Kok, M MensinkHigh dietary protein intake, reducing or eliciting insulin resistance?Eur J Clin Nutr.(2014 Sep)
- ^Zachary BloomgardenDiabetes and branched-chain amino acids: What is the link?J Diabetes.(2018 May)
- ^Pinar Kozan, Jackson C Blythe, Jerry R Greenfield, Dorit Samocha-BonetThe Effect of Buffering High Acid Load Meal with Sodium Bicarbonate on Postprandial Glucose Metabolism in Humans-A Randomized Placebo-Controlled StudyNutrients.(2017 Aug 11)
- ^Harris SS, Dawson-Hughes BNo effect of bicarbonate treatment on insulin sensitivity and glucose control in non-diabetic older adultsEndocrine.(2010 Oct)
- ^I A Osuna-Padilla, G Leal-Escobar, C A Garza-García, F E Rodríguez-CastellanosDietary Acid Load: mechanisms and evidence of its health repercussionsNefrologia (Engl Ed).(Jul-Aug 2019)
- ^Guido Maarten Kamphuis, Jons Wouter van Hattum, Prim de Bie, Bhaskar K SomaniMethod of alkalization and monitoring of urinary pH for prevention of recurrent uric acid urolithiasis: a systematic reviewTransl Androl Urol.(2019 Sep)
- ^Zuckerman JM, Assimos DGHypocitraturia: pathophysiology and medical managementRev Urol.(2009 Summer)